
Is Fda S Special 510 K Program Appropriate For Your Medical

Fda Issues Draft Guidance For The Safer Technologies Program For
Design Control Fda Requirements

Fda Design Control Guidance For Medical Devices Manufacturers Rx
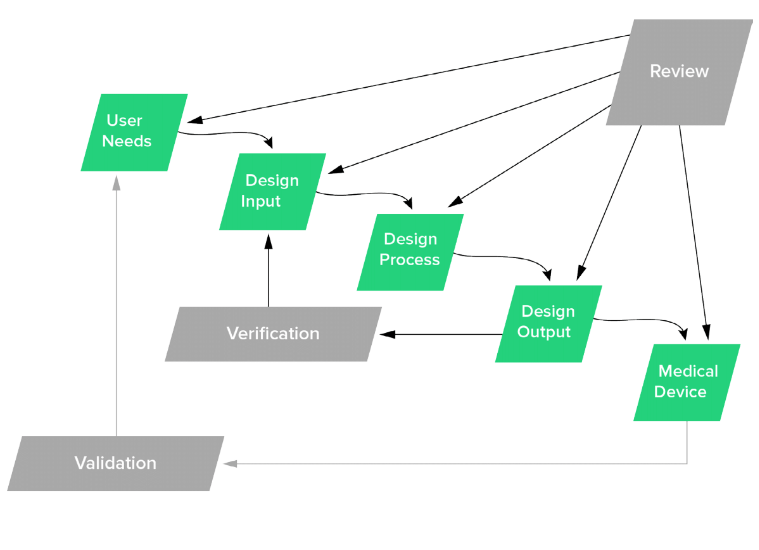
The Ultimate Guide To Design Controls For Medical Device Companies
Design Controls Fda

Design Validation And Regulatory Requirements Medical Design Briefs

Basic Qms Compliance Intro To Quality Management System Fda

Design Control Sop For Medical Devices Industry Under Gmp

Fda S New Quality Agreement Guidance What It Says And What It

Supplier Management For Medical Device Manufacturers

Fda Design Control Guidance For Medical Devices Perforce
Application Of Design Controls To Design Process Design Review

Perforce Software On Twitter Need To Follow Fda Design Control

Understanding Fda Thinking Risk Benefit Determinations In Design

Design Controls An Overview Sciencedirect Topics
Https Www Advamed Org Sites Default Files Resource Software In Medical Devices Module 1 Pdf

Fda Design Control Guidance For Reviews And Validation Of Designs

Medical Device Design And Development Ppt Download
Medical Device Design And Developement Validation Regulation
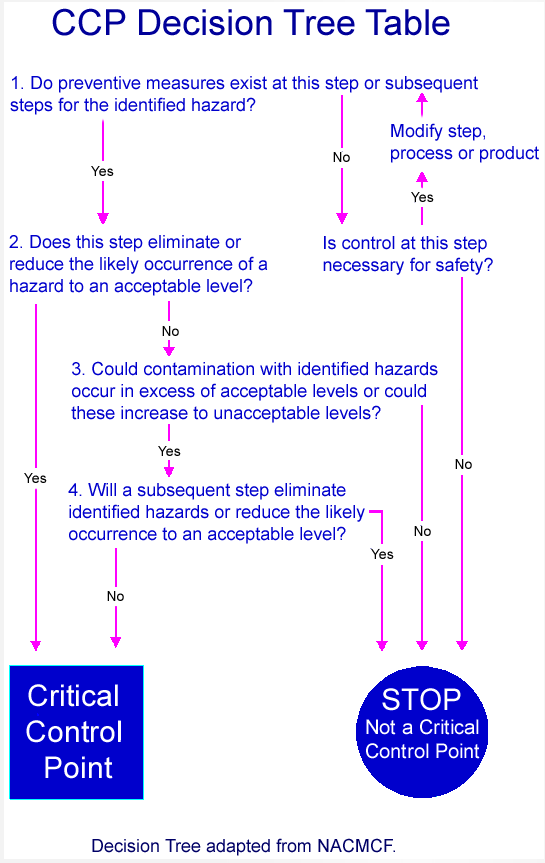
Guidance For Industry Juice Hazard Analysis Critical Control

Medical Devices Biomedical Engineering Libguides At City
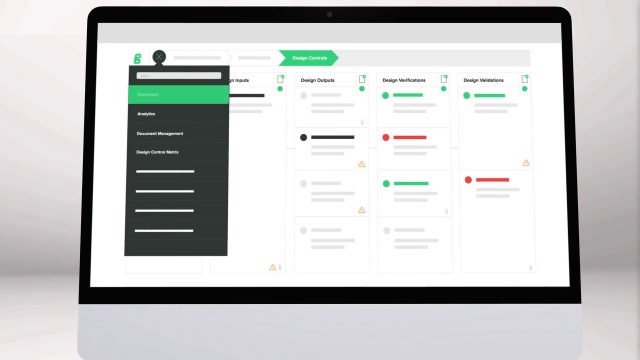
The Ultimate Guide To Design Controls For Medical Device Companies
Medical Device Process Validation What You Need To Know
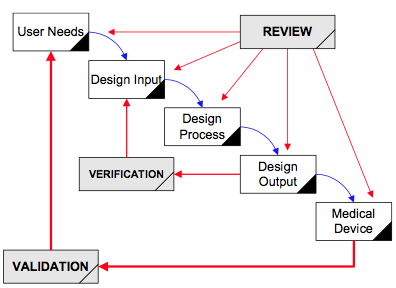
Implementing Design Controls Medical Device Academy 10 Steps

Design Control Fda Requirements

Design Control Fda Requirements
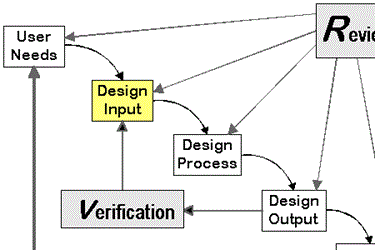
The Art Of Medical Device Design Inputs

Sally Mcarthur On Twitter R D Design Controls For Qms Of

Trends In Medical Device Recalls Medtech Intelligence
Design Verification And Design Validation What S The Difference

Design Controls Fda

Fda Outlines Plans For Medical Devices With Artificial Intelligence

Pdf Using Quality System Regulations And Fda Design Control

It S Easy To Turn Money Into Technology It S Not So Easy To Turn
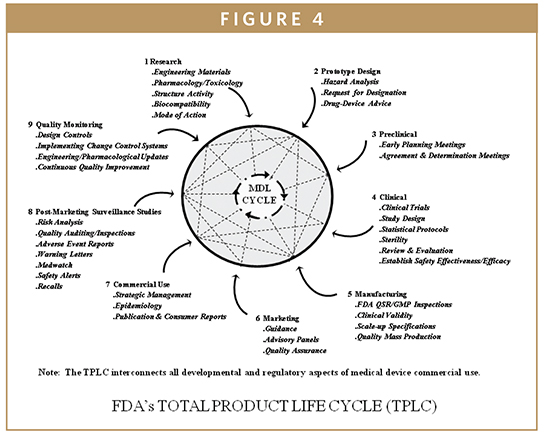
Biomarkers Fda S Design Control Requirements For Biomarkers In

Design Controls Addressing Changes In Iso 13485 Medical Design

Fda Guidance On Gene Therapies Development And Manufacturing

How To Prepare Your Design History File Dhf For An Fda Inspection
No comments:
Post a Comment